Blog
Solntrey receives a quick FDA designation for Auraza wound gel to treat kalphylaacism ulcers
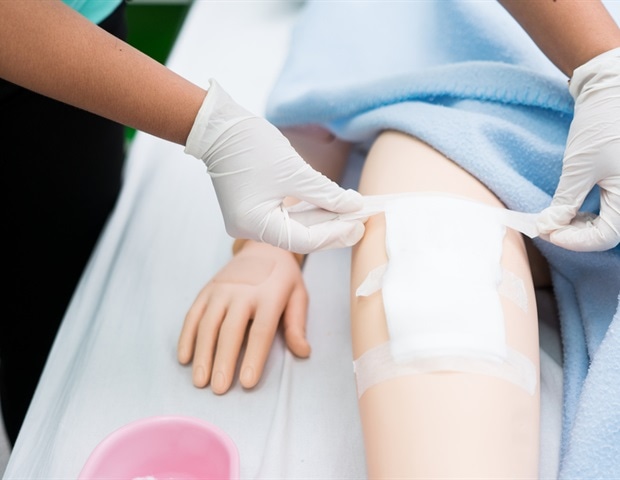
Solascure Ltd (Solascure), a biotechnology company, developing innovative treatment to transform chronic wound healing, today announced that the American food and medicine administration (FDA) has granted a quick designation to the tested gel auraza wound (AWG), in the treatment of patients with kalcyphylaxis ulcers. The designation recognizes the potential of AWG in the treatment of this new indication, expanding its use in healing chronic wounds, while accelerating the speed at which new therapy can be available to patients.
Calkiphylaxis is a rare but serious condition including calcification of small blood vessels in fat and skin. It leads to blood clots, painful skin ulcers and can go to severe infections and sepsis. Although most often associated with the final kidney disease, the condition may also occur in people with normal kidney function. Thanks to limited treatment options and a high annual mortality rate, AWG offers an alternative cleansing solution, which potentially reduces the risk of infection and sepsis, opening patients’ treatment options, which were previously considered too fragile to receive the current standard of care and improve the results in this sensitive population.
AWG is a hydrogel tarumaza, a recombinant enzyme originally isolated from medical worms, which selectively attacks fibrin, collagen and elastin in wounds to promote healing by cleansing and preparing wounds. It is currently in clinical trials of phase II in the treatment of venous leg ulcers, after determining the proof of the concept, a strong safety profile and painless use.
Considering the unsatisfied medical need and bad results for patients with calcylaxis, the granting of a fast FDA track is a significant milestone. It not only reflects the promise of the Auraza wound gel in the treatment of kalphylaactic ulcers, but also adds a new indication, increasing its potential to help more patients, thus opening an even larger target market for dissolving. “
David Fairlamb, Development Director, Soloszczel